Microfluidics
✓ Precise control of fluids at the microscale
✓ Enable lab-on-a-chip diagnostics & drug-screening
✓ Laminar flow, diffusion-based mixing, and miniaturized systems
Microfluidics is a scientific discipline that deals with the manipulation of small quantities of fluids. Microfluidic devices have small features and channels that are on the order of 10s to 100s of microns in diameter, and which support the flow of fluid volumes of microliters (10⁻⁶ L) to picoliters (10⁻¹² L). The behavior of fluids at this scale is very different to what we are used to at the macroscale. At the scale of microfluidic devices, the effect of viscosity and capillarity are much more important, and the effects of gravity or inertia are much less important than on the macroscale. Because of this, turbulences are minimal, and the fluid flow is very predictable laminar flow.
Laminar vs Turbulent Flow
Whether fluid flow is laminar or turbulent is determined by the Reynolds Number, Re, which is a dimensionless number that can be calculated with the following formula:
For low values of Re, typically less than 2,000, the flow through a tube is laminar.
For high values of Re, typically more than 2,200, the flow through a tube is turbulent.
In microfluidic devices, the diameter of the channels is typically 10 micron (10-5 m) to several hundred micron (10-4 m), i.e., the Reynolds number is low, and the flow is therefore mostly laminar. This changes also how fluids are mixed because chaotic mixing does not occur at the microscale. Instead, mixing occurs through diffusion between different streams.
Our team of experts...
Founder
Phone: +1 (609) 532-9744
Email: oliver@bmseed.com
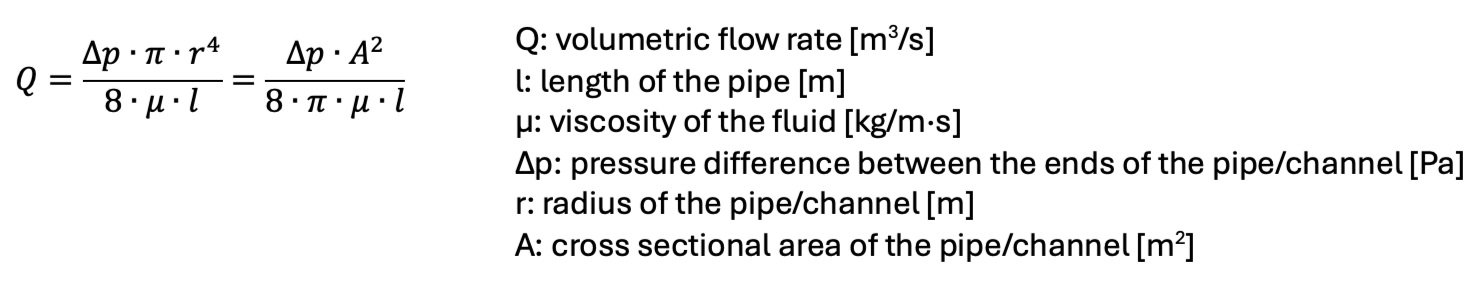
Volumetric Flow Rate Q
The volumetric flow rate Q through a channel or pipe is given by the Hagen-Poiseuille equation:
The flow rate through a channel increases with the radius to the power of 4, i.e., the flow rates in microfluidic devices are very low but can be precisely controlled. For a given microfluidic device (l, r, A are constant) and fluid (μ is constant), the flow rate through microchannel only depends on the pressure difference between the outlet and the inlet of the channel. Controlling the pressure difference that drives the fluid through the channel is therefore a critical feature of microfluidic systems. The three most common methods to control the pressure difference, hence the flow rate through the channel, are:
1. Hydrostatic Pressure
This is the simplest way to generate controlled flows in a microfluidic system. The pressure difference is created by varying the height difference between the microfluidic channel and the reservoirs. For aqueous liquids, a difference of 1 cm corresponds to 1mbar hydrostatic pressure. The resolution of this technique to create pressure is about 0.1 mbar on the low end (1 mm height difference) and about 100 mbar (1 m height difference).
2. Syringe Pump
A syringe pump uses a precisely controlled stepper motor to drive a mechanical system that pushes the plunger of a syringe at a constant and programmable rate, resulting in a highly accurate and continuous flow of fluid. The accuracy of the flow rate depends on the step size of the motor (the minimum distance the plunger moves in one step of the motor) and the dimension of the syringe (smaller syringes have a higher accuracy than larger syringes, but the maximum flow rate is lower). The main advantage of syringe pumps is their capability to control the flow across microchannels independently of fluidic resistance because the hydrodynamic pressure increases with resistance to flow. The main drawback of syringe pumps is that the flow is pulsatile because at each step of the motor, the initial pressure is highest immediately after the motion is completed. However, there are methods to smoothen the swings in flow rates using syringe pumps. Ask BMSEED for information.
3. Peristaltic Pump
A peristaltic pump, also known as a roller pump, uses a “squeezing and releasing” action on a flexible tube to move fluid, offering a unique combination of fluid isolation, gentle handling, and relatively simple operation. The fluid is contained within a flexible tube or hose, typically made of an elastomeric material, fitted inside the pump casing. This tube is the only part of the pump that comes into contact with the fluid. The pump features a rotor with a number of rollers, shoes, or wipers attached to its outer circumference. As the rotor turns, these rollers or shoes compress the flexible tube against a track or the pump housing. This compression creates a temporary seal, “occluding” the tube and pinching off a segment of fluid.
The rotating motion of the rollers moves this point of compression along the length of the tube. The fluid trapped ahead of the compression point is pushed forward towards the pump outlet. Once the roller passes and the compression is released, the elasticity of the tube causes it to rebound to its original shape. This creates a vacuum in the tube behind the moving compression point, drawing more fluid into the pump inlet. The continuous rotation of the rotor and rollers creates a cycle of compression and relaxation, resulting in a continuous flow of fluid through the tube.
A major benefit of a peristaltic pump is that the simple design eliminates the need for valves, seals, and glands that can wear out, leak, or clog, reducing maintenance requirements. The major drawback of peristaltic pumps is the pulsating flow. As for syringe pumps, there are methods to reduce the swings in flow rates. In addition, the flow rate accuracy is lower than for syringe pumps and the flexible tubing inside the pump needs to be replaced periodically due to wear and tear.
4. Pressure Generators
A pressure generator for microfluidic applications works by applying controlled pneumatic (gas) pressure to a liquid reservoir connected to the microfluidic device. This pressure difference drives the fluid flow through the microchannels.
The liquid to be introduced into the microfluidic device is contained within a sealed reservoir. The pressure generator is connected via a pneumatic tube to the air-filled space above the liquid inf the reservoir. A precise pressure regulator is used to control the amount of pressure applied to the reservoir. The applied pressure in the reservoir pushes down on the liquid surface, and since liquids are generally incompressible, this pressure is transmitted through the liquid, forcing it to flow out of the reservoir and into the connected microfluidic channels.
The flow rate within the microfluidic channels is directly proportional to the applied pressure difference between the reservoir and the outlet of the microfluidic device, as well as the fluidic resistance of the channels themselves. Higher pressure results in a higher flow rate, and vice versa. More complex pressure-driven systems can incorporate valves to switch between multiple reservoirs containing different fluids or to control the timing and direction of flow. Incorporation of flow sensors in a feedback loop can help control the pressure and maintain the desired flow rate. The major benefits of pressure generators to control flow rate in a microfluidic device is that they provide pulseless flow, unlike syringe pumps and peristaltic pumps, at a fast response time. The major drawback of pressure generators is that the flow rate is only indirectly controlled. Direct control of flow rate requires closed-loop feedback with a flow sensor, which adds to the complexity of the method.
Ask BMSEED which type of flow control is right for you!
Our team of experts...
Founder
Phone: +1 (609) 532-9744
Email: oliver@bmseed.com
Applications
Microfluidic devices have a wide array of applications across various scientific and industrial fields. Here are a few key examples:
1. Biomedical Diagnostics and Healthcare:
Point-of-Care Diagnostics: Development of portable and rapid diagnostic devices for diseases (e.g., infectious diseases, cancer, genetic disorders), pregnancy tests, and blood glucose monitoring.
Disease Modeling: Creating organ-on-a-chip systems that mimic the function of human organs to study diseases like cancer and test drug efficacy in a more physiologically relevant environment than traditional cell cultures or animal models.
Single-Cell Analysis: Studying individual cells to understand cellular heterogeneity, gene expression, and responses to stimuli.
Drug Delivery: Developing microfluidic systems for precise and targeted drug delivery, as well as for creating drug carriers and controlling drug release rates.
Circulating Tumor Cell (CTC) Detection: Isolating and analyzing rare CTCs from blood samples for cancer diagnosis and prognosis.
Gene Delivery: Efficiently transferring genetic material into cells.
Fertility Aid: Applications in sperm selection and semen analysis.
2. Pharmaceutical Industry and Drug Discovery:
High-Throughput Drug Screening: Automating and miniaturizing drug screening processes to test the effects of numerous compounds on cells or disease models.
Drug Toxicity Screening: Evaluating the safety and efficacy of new drug candidates.
Personalized Medicine: Developing diagnostic and therapeutic tools tailored to individual patient needs.
Nanoparticle Synthesis: Creating nanoparticles for drug delivery and imaging with precise control over size and properties.
3. Environmental Monitoring:
Pollutant Detection: Developing portable and cost-effective devices for on-site detection of contaminants in water, air, and soil (e.g., heavy metals, pesticides, pathogens).
Water Quality Monitoring: Real-time monitoring of water sources for pollutants and microorganisms.
Air Quality Analysis: Detecting and quantifying airborne pollutants.
4. Chemical Synthesis and Analysis:
Microreactors: Performing chemical reactions in miniaturized channels with precise control over reaction conditions (temperature, mixing, residence time), leading to higher yields and purity.
Continuous Flow Synthesis: Enabling continuous production of chemicals in small amounts with consistent quality.
Nanomaterial Synthesis: Controlled production of nanoparticles and other nanomaterials.
Analytical Chemistry: Integrating multiple analytical functions onto a single chip for faster and more efficient analysis with reduced sample consumption (e.g., DNA and protein analysis).
5. Fundamental Research:
Cell Biology Studies: Investigating cell behavior, cell-cell interactions, and cellular responses to microenvironmental cues.
Fluid Dynamics: Studying fluid flow and behavior at the microscale (e.g., laminar flow).
Biomaterials Research: Developing and testing new biomaterials for tissue engineering and other applications.
These are just a few examples, and the field of microfluidics is constantly evolving with new applications emerging in diverse areas of science and technology.